A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes†
Vsevolod V. Rostovtsev Dr.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorLuke G. Green Dr.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorValery V. Fokin Prof.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorK. Barry Sharpless Prof.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorVsevolod V. Rostovtsev Dr.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorLuke G. Green Dr.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorValery V. Fokin Prof.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorK. Barry Sharpless Prof.
Department of Chemistry and the Skaggs Institute for Chemical Biology The Scripps Research Institute, BCC-315 10550 N. Torrey Pines Rd., La Jolla, CA 92037 (USA) Fax: (+1) 858-784-7562
Search for more papers by this authorWe thank the National Institute of General Medical Sciences, the National Institutes of Health (GM 28384), the National Science Foundation (CHE-9985553), and the W. M. Keck Foundation for financial support. We also thank Dr. F. Himo, Prof. L. Noodleman, Prof. Flavio Grynszpan, and Prof. M. G. Finn for helpful discussions.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z19191_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a R. Huisgen in 1,3-Dipolar Cycloaddition Chemistry ( ), Wiley, New York, 1984, pp. 1–176;
- 1b
A. Padwa in Comprehensive Organic Synthesis, Vol. 4 ( ), Pergamon, Oxford, 1991, pp. 1069–1109;
10.1016/B978-0-08-052349-1.00116-5 Google Scholar
- 1c for a review of asymmetric 1,3-dipolar cycloaddition reactions, see K. V. Gothelf, K. A. Jorgensen, Chem. Rev. 1998, 98, 863–909;
- 1d for a review of synthetic applications, see J. Mulzer, Org. Synth. Highlights 1991, 77–95.
- 2
- 2a W.-Q. Fan, A. R. Katritzky in Comprehensive Heterocyclic Chemistry II, Vol. 4 ( ), Pergamon, Oxford, 1996, pp. 101–126;
- 2b R. N. Butler in Comprehensive Heterocyclic Chemistry II, Vol. 4 ( ), Pergamon, Oxford, 1996, pp. 621–678;
- 2c K. Banert, Chem. Ber. 1989, 122, 911–918.
- 3
- 3a R. Huisgen, Pure Appl. Chem. 1989, 61, 613–628;
- 3b R. Huisgen, G. Szeimies, L. Moebius, Chem. Ber. 1967, 100, 2494–2507;
- 3c W. Lwowski in 1,3-Dipolar Cycloaddition Chemistry, Vol. 1 ( ), Wiley, New York, 1984, chap. 5;
- 3d J. Bastide, J. Hamelin, F. Texier, V. Q. Ven, Bull. Soc. Chim. Fr. 1973, 2555–2579; J. Bastide, J. Hamelin, F. Texier, V. Q. Ven, Bull. Soc. Chim. Fr. 1973, 2871–2887.
- 4 Although applications which utilize the unique reactivity offered by the azide group itself are rare, delightful exceptions can be found the following works:
- 4a P. Desai, K. Schildknegt, K. A. Agrios, C. Mossman, G. Milligan, J. Aube, J. Am. Chem. Soc. 2000, 122, 7226–7232;
- 4b K. Banert, Targets Heterocycl. Syst. 1999, 3, 1–32; K. Banert, Liebigs Ann./Recl. 1997, 2005–2018;
- 4c W. H. Pearson, W.-K. Fang, Isr. J. Chem. 1997, 37, 39–46;
- 4d J. Cao, M. C. T. Fyfe, J. F. Stoddart, J. Org. Chem. 2000, 65, 1937–1946;
- 4e B. Carboni, A. Benali, M. Vaultier, J. Org. Chem. 1993, 58, 3736–3741, and references therein.
- 5
H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 1198–1220;
Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 6 Organic azides, particularly in the aliphatic series, are exceptionally stable toward the common reactive chemicals on the Earth's surface, which range from dioxygen and water to the aqueous solutions of highly functionalized organic molecules which make up living cells: E. Saxon, C. R. Bertozzi, Science 2000, 287, 2007–2010; K. L. Kiick, E. Saxon, D. A. Tirrel, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2002, 99, 19–24.
- 7
In fact, it was the razor sharp reactivity window for this cycloaddition process which spawned our “in situ click chemistry” ideas—an approach which recently resulted in the discovery of the most potent noncovalent inhibitor of acetylcholinesterase known to date: W. G. Lewis, L. G. Green, F. Grynszpan, Z. Radic, P. R. Carlier, P. Taylor, M. G. Finn, K. B. Sharpless, Angew. Chem. 2002, 114, 1095–1099;
Angew. Chem. Int. Ed. 2002, 41, 1053–1057.
10.1002/1521-3773(20020315)41:6<1053::AID-ANIE1053>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 8 Unless the acetylene component is attached to an electron-withdrawing group such as a carbonyl or perfluoroalkyl group: J. Bastide, O. Henri-Rousseau, Bull. Chim. Soc. Fr. 1973, 2294–2296; N. P. Stepanova, N. A. Orlova, V. A. Galishev, E. S. Turbanova, A. A. Petrov, Zh. Org. Khim. 1985, 21, 979–983; N. P. Stepanova, V. A. Galishev, E. S. Turbanova, A. V. Maleev, K. A. Potekhin, E. N. Kurkutova, Yu. T. Struchkov, A. A. Petrov, Zh. Org. Khim. 1989, 25, 1613–1618; D. Clarke, R. W. Mares, H. McNab, J. Chem. Soc. Perkin Trans. 1 1997, 1799–1804.
- 9 P. Zanirato, J. Chem. Soc. Perkin Trans. 1 1991, 2789–2796; D. J. Hlasta, J. A. Ackerman J. Org. Chem. 1994, 59, 6184–6189; C. A. Booth, D. Philp, Tetrahedron Lett. 1998, 39, 6987–6990; S. J. Howell, N. Spencer, D. Philp, Tetrahedron 2001, 57, 4945–4954; W. L. Mock, T. A. Irra, J. P. Wepsiec, M. Adhya, J. Org. Chem. 1989, 54, 5302–5308; W. L. Mock, Top. Curr. Chem. 1995, 175, 1–24; J. Chen, J. Rebek, Jr., Org. Lett. 2002, 4, 327–329; J. W. Wijnen, R. A. Steiner, J. B. F. N. Engberts, Tetrahedron Lett. 1995, 36, 5389–5392; M. P. Repasky, W. L. Jorgensen, Faraday Discuss. 1998, 110, 379–389.
- 10 While this manuscript was in preparation, an independent account of copper-catalyzed synthesis of 1,4-triazoles from azides and terminal acetylenes on solid support was reported: C. W. Tornøe, C. Christensen, M. Meldal, J. Org. Chem. 2002, 67, 3057.
- 11 For a review of reactions of L-ascorbic acid with transition metals, see M. B. Davies, Polyhedron 1992, 11, 285–321, and references therein; redox properties of ascorbic acid are summarized in C. Creutz, Inorg. Chem. 1981, 20, 4449.
- 12 The starting materials do not need to be dissolved in the reaction solvent. The reaction seems to proceed just as efficiently as long as adequate stirring is maintained.
- 13 Amazingly, even Cu0 can be used as a source of the catalytic species. Although these reactions may take longer to proceed to completion, the experimental procedure is exceedingly simple. For example, bis-triazole shown in entry 2 (Table 1) was obtained in quantitative yield after stirring the corresponding azide and acetylene components for 24 h with about 1 g of coiled copper metal turnings. The turnings were removed at the end of the reaction, and the pure white product was collected by simple filtration.
- 14 CCDC 186236 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 15 For a recent summary of the reactions of copper(I) complexes with dioxygen, see S. Schindler, Eur. J. Inorg. Chem. 2000, 2311–2326 and A. G. Blackman, W. B. Tolman in Structure and Bonding, Vol. 97 ( ), Springer, Berlin, 2000, pp. 179–211.
- 16 For example, ethyl propiolate and benzylazide furnished the corresponding 1,4-triazole in 55 % yield when this procedure was used, but only a trace amount of the product was obtained with one equivalent of triethylamine and without exclusion of oxygen.
- 17 G. van Koten, J. G. Noltes in Comprehensive Organometallic Chemistry, Vol. 2 ( ), Pergamon, Oxford, 1982, chap. 14, p. 720.
- 18 F. Himo, T. Lovell, V. Rostovtsev, V. V. Fokin, K. B. Sharpless, L. Noodleman, unpublished results.
- 19 M. P. Doyle, M. A. McKervey, T. Ye in Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1997, pp. 163–248.
- 20
The reaction proceeded equally well even in human plasma (protein loading 65–85 mg mL−1, Cazide=Calkyne=5 mM; C
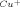 =100 μM), which indicates that the copper species remained available for the catalysis despite being heavily bound to plasma proteins.
=100 μM), which indicates that the copper species remained available for the catalysis despite being heavily bound to plasma proteins.



41:14<>1.0.co;2-b.cover.gif)
